kava & kratom
cinna-mints
cinna-mints

Are you 18 or older and ready to LIVE FULLY?
Sorry, the content of this store can't be seen by a younger audience. Come back when you're ready.







Whole-leaf kratom
All-natural kratom can support the response to occasional pain and inflammation.†
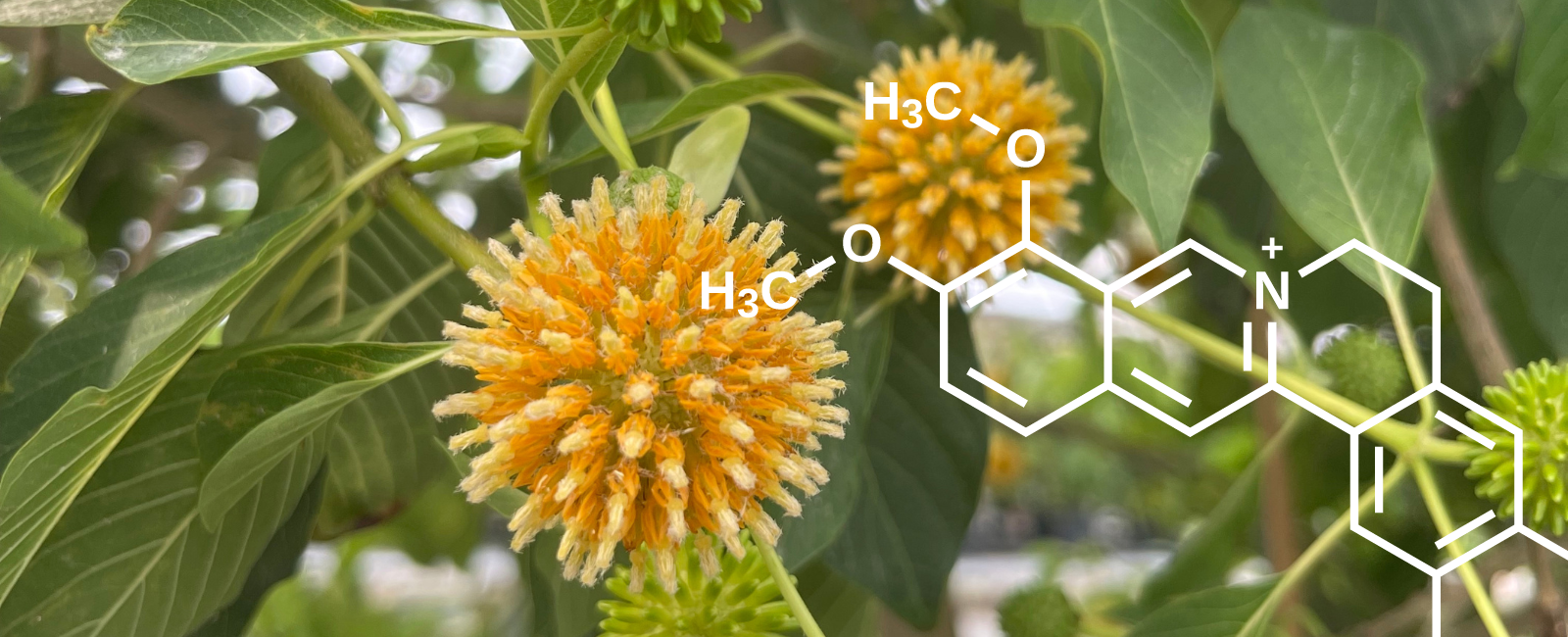
While kratom is enjoyed as Pure Veins, ETHA goes the extra mile to create Premium Kratom options too. Every kratom harvest contains different concentrations of alkaloids, the source of kratom's benefits. We meticulously measure these alkaloids and blend different harvests to craft unique recipes we call our Premium Kratom. Our dedication ensures consistency in every product, guaranteeing you remarkable results.
★★★★★"I've had over 28 orthopedic surgeries for my sport. Kratom is a miracle plant that has really helped me."
Danny Way
Legendary Skateboarder
★★★★★"I've found an all-natural alternative to coffee. It's called kratom!"
Monica Richards
Sustainable Living Coach
★★★★★"We both suffer from chronic pain. Kratom gave us our lives back. We are proud to have the opportunity to share this amazing plant with you."
Alexander & Victor
ETHA Co-Founders
★★★★★"I suffer from chronic, lower back pain. I am so happy to have discovered ETHA kratom."
Tyler Thomas
Chef & Owner - The American Restaurant
Legendary Skateboarder, Danny Way, uses ETHA Kratom Mints and Kratom Hazelnut Creamer Shots to continue to train and ride.
Sustainable Living Coach, Monica Richards' favorite ETHA products are Maeng Da Kratom Tablets and MidDay Gold Kratom Tablets.
Chef and Owner of The American Restaurant, Tyler Thomas, and his wife, Heather, manage their pain with ETHA SunRise Kratom.